|
Introduction:
Halobacteria is an extremophile that lives in very salty environments. One of the natural environments of Halobacteria is the Great Salt Lake in Utah, NV. This lake’s salinity varies with the seasons and we wondered what salinity is optimal for Halo growth. We tested how Halo grows in media with salinities of 2.3M, 2.8M, 3.3M, 3.8M, and 4.3M. We performed the experiment in flacon tubes using an incubator and in flasks on a spinning plate to compare the procedures and determine what could be replicated in a high school classroom.
Procedure Using Falcon Tubes
- Add 5.0 mL of media for the first concentration to four different falcon tubes.
- Repeat this process for each concentration of media.
- Add 100.0 μL of Halobacterium to each Falcon tube EXCEPT THE CONTROL using a micropipet.
- Dispose of pipet tip and micropipet tips after putting together all four samples.
- Make sure Falcon tube caps are securely fastened, pressing cap to its second stop.
- Place inoculated, labeled Falcon tubes in incubating rack. The samples will incubate for 48 hours at 37° C & 220 rpm.
- Record the date and time the samples are placed in the incubator.
48 Hours Later
- Obtain your four samples from incubator.
- Pipet 1 mL from each sample into a clean cuvette.
- Measure the cell density of each sample using the spectrophotometer.
- Set spectrophotometer reading to absorbance and the wavelength to 600 nm.
- Use Kim wipes when handling cuvettes.
- To zero spectrophotometer insert provided blank, press blank.
- Insert cuvette with sample, record absorbance reading.
- Repeat steps a – d for each sample.
- Record data in your data table.
- Clean up your lab space and equipment.
- Rinse cuvettes thoroughly three times with hot water.
- Rinse three more times with deionized water.
- Return equipment to proper locations (RETURN SAMPLES TO INCUBATOR)
Variations to Procedure when Using Flasks
- Add 15 mL instead of 5 mL of media for first concentration to four different flasks.
- Add 300 µL instead of 100 µL of Halobacterium to each flask except the control.
- Cover flasks with aluminum foil
- Cut four “X’s” in an 8” by 8” piece of cardboard and use this to hold the flasks in place on top of the spinning plates. Tape the cardboard to some sort of support so the flasks are stable.
- Turn on the stir plates to lowest level.
- Record the date and time the samples are placed in the incubator.
48 Hours Later
- Pipet 1 mL from each sample into a clean cuvette.
- Measure the cell density of each sample using the spectrophotometer.
- Set spectrophotometer reading to absorbance and the wavelength to 600 nm.
- Use Kim wipes when handling cuvettes.
- To zero spectrophotometer insert provided blank, press blank.
- Insert cuvette with sample, record absorbance reading.
- Repeat steps a – d for each sample.
- Record data in your data table.
- Clean up your lab space and equipment.
- Rinse cuvettes thoroughly three times with hot water.
- Rinse three more times with deionized water.
- Return equipment to proper locations
120 Hours after beginning experiment
Repeat steps 1-5 performed after 47 hours with the exception of returning samples to stir plates.
Setup
| Flasks |
Falcon Tubes (to be placed in Incubator) |
 |
 |
Results
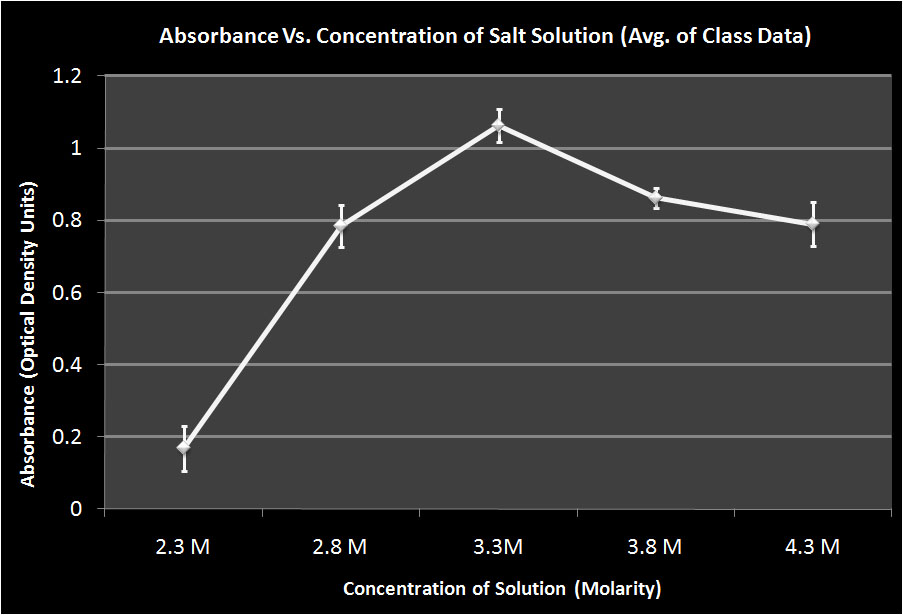
Falcon Tubes
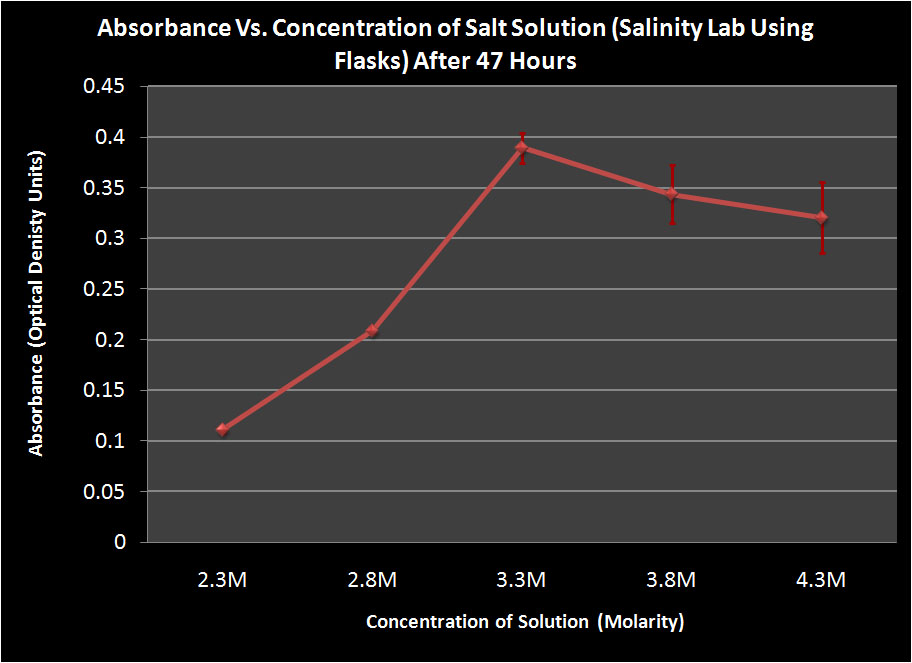
Flasks
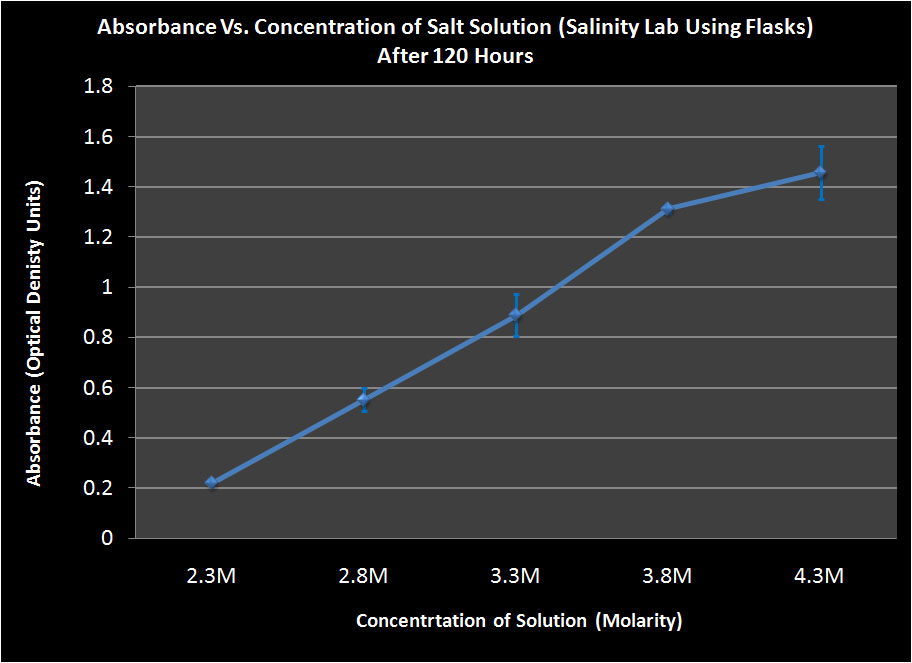
Conclusion
We found that after 47 hours, the Halo had grown best in the 3.3M solution in both the flasks and the falcon tubes. However, after 120 hours, the Halo grown in the 4.3M solution showed the most growth. We are unsure as to why the Halo grew the most in a 3.3M solution after 47 hours because halo grows the best at higher salinities. This may be partly explained by the bacteria being in a more stressed state in the 3.3M solution and this throws off their growth cycle. When stressed, some organisms reproduce more rapidly in hopes of maintaining a large population. However, the Halo may not have been able to maintain this rapid reproduction and began to die off. This theory is supported by the fact that the most dead cells were found at the bottom of the 3.3M tube after 120 hours. This suggests that the cells grew rapidly but then began to die off and their population could not maintain such a high growth rate.
|



