|
Further Investigation: Copper Absorbance Spectrum Lab
Purpose:
The purpose of this lab was to observe two different environmental factors. One was to see the effect of copper on the growth of Halo and the second was to see whether these Halo solutions with copper would show differences in growth if exposed to light or kept in the dark.
Procedure:
Using gloves, a lab coat, and eye protection, 5mL of 1.2M CuSO4 stock solution was made. The CuSO4 solution was then vortexed until all of the crystals were dissolved. 10 mL of regular CM was transferred into 5 separate Falcon tubes. The tubes were labeled 0mM, 0.7 mM, 0.85 mM, 1.0 mM, and 1.2 mM and the appropriate amount of copper sulfate solution was placed into each tube to match labeled dilution.
200 µL of water was placed into the first slot of a quartz cuvette, then 200 µL of each solution was placed into the subsequent cuvette slots in the order of 0 mM, 0.7 mM, 0.85 mM, 1.0 mM, 1.2 mM. After placing the cuvettes in the UD 800 spectrophotometer, the full spectrum of each sample was measured, using the 8th slot (air), as the blank.
Half of the left over samples in the Falcon tubes, including the copper sulfate solution, was transferred to new falcon tubes with corresponding labels. One set of the samples were placed in sunlight and the other was set in the darkness of a refrigerator. The initial spectrum values using the data from the spectrophotometer were graphed. 48 hours later all of the samples were retrieved from the window sill (sun) and refrigerator(dark).
13 microcentrifuge tubes were then labeled with the numbers 1 through 13. Using a micropipette, 250 µL of each sample were transferred to the microcentrifuge tubes following the chart:
#1 = CuSO4 1.2M (dark) |
#7 = CuSO4 1.2M (sun)
|
#2 = 0.0 mM (dark) |
#8 = 0.0 mM (sun) |
#3 = 0.7 mM (dark) |
#9 = 0.7 mM (sun) |
#4 = 0.85 mM (dark) |
#10 = 0.85 mM (sun) |
#5 = 1.0 mM (dark) |
#11 = 1.0 mM (sun) |
#6 = 1.2 mM (dark) |
#12 = 1.2 mM (sun) |
After sealing the microcentrifuge tubes 100µL of Halo and 100 µL of deionized water was placed in the same cuvette in order to dilute the Halo solution by two. The OD of this diluted solution was measured and 250 µL of the original Halo solution was placed in the falcon tubes with different concentrations of copper. Halo was not placed in the stock copper solutions. The tubes were sealed to the second stop and inverted to mix and 100µL of each sample were placed in cuvettes.
The OD was then measured and the samples with Halo were placed in an incubator at 37ºC and 220 rpm. Using a micropipette 200 µL of the samples were transferred from the microcentrifuge tubes into quartz cuvettes. The order of the samples in the cuvette should be the same as the order of the samples the first time the spectrophotometer was used. The spectrophotometer was once again blanked on air (cuvette slot 8). The spectrums were measured for each sample and graphs.
Results:
This lab was repeated three times because the results were counter-intuitive. We had expected the light to degrade the copper within the samples left in the light and thus hypothesized that Halo would grow in light samples more than those samples left in the dark.
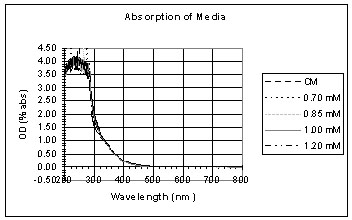
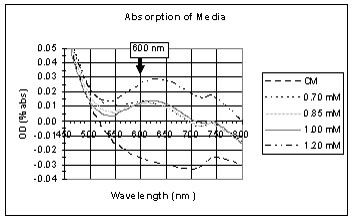
These graphs show the spectrum of the copper solutions before being placed in the light and dark.
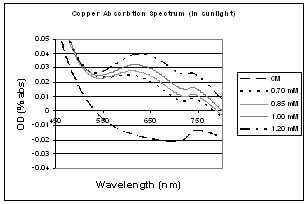
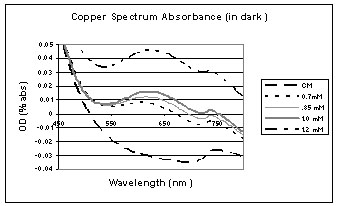
These graphs show the copper spectrums after being left in the sun or freezer after 48 hours.
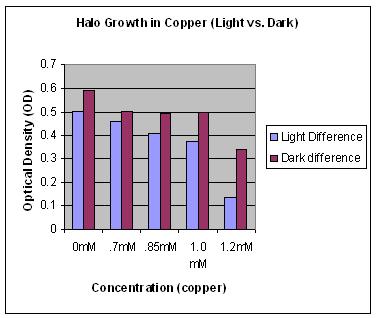
This graph shows that halo grew the best in a 0 mM concentration of copper in the dark. This is because a bigger "dark difference" (the difference between the beginning OD and the end OD) means that there is more halo growth.
[back to top]
|